Informations sur le produit
- 1,1′,1′′-Silylidynetris[benzene]
- Benzene, 1,1',1''-silylidynetris-
- Benzene, 1,1′,1′′-silylidynetris-
- Ls 6390
- Nsc 12565
- Silane, triphenyl-
- Sit 8665
- Trifenilsilano
- Triphenylhydrosilane
- Triphenylsilan
- Voir d'autres synonymes
- Triphenylsilyl
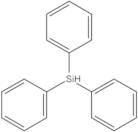
- Triphenylsilane
Tri-substituted Silane Reducing Agent
Organosilanes are hydrocarbon-like and possess the ability to serve as both ionic and free-radical reducing agents. These reagents and their reaction by-products are safer and more easily handled and disposed than many other reducing agents. The metallic nature of silicon and its low electronegativity relative to hydrogen lead to polarization of the Si-H bond yielding a hydridic hydrogen and a milder reducing agent compared to aluminum-, boron-, and other metal-based hydrides. A summary of some key silane reductions are presented in Table 1 of the Silicon-Based Reducing Agents brochure.
Triphenylsilane; Triphenylsilanlyl hydride
More effective radical-based reagent for reduction of organic halides than the trialkylsilanesCompares well with tri-n-butyltin hydride in reduction of enones to ketonesShows good selectivity in the reduction of cyclic hemiacetalsConverts O-acetyl furanoses and pyranoses to deoxy sugarsExtensive review of silicon based reducing agents: Larson, G.; Fry, J. L. "Ionic and Organometallic-Catalyzed Organosilane Reductions", Wipf, P., Ed.; Wiley, 2007
Propriétés chimiques
Question d’ordre technique sur : 3H-SIT8665.0 TRIPHENYLSILANE
Si vous souhaitez demander un devis ou passer commande, veuillez plutôt ajouter les produits souhaités à votre panier, puis demander un devis ou passer commande à partir de votre panier. C'est une méthode plus rapide, plus économique, et vous pourrez bénéficier des remises disponibles ainsi que d'autres avantages