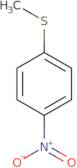
4-Nitrothioanisole
CAS: 701-57-5
Rif. 3D-AAA70157
| 1g | Fuori produzione | ||
| 10g | Fuori produzione | ||
| 25g | Fuori produzione | ||
| 50g | Fuori produzione | ||
| 100g | Fuori produzione | ||
| 250g | Fuori produzione | ||
| 100mg | Fuori produzione |
Informazioni sul prodotto
- p-Nitrophenyl methyl sulfide
- 1-(Methylsulfanyl)-4-Nitrobenzene
4-Nitrothioanisole is a white solid that has a molecular weight of 119.07 grams per mole. It has optical properties and is a hydrogen bond donor. 4-Nitrothioanisole can be used as an intermediate in the synthesis of other compounds, such as dinitrobenzene or 2,4-dinitrotoluene. The molecule can form cavities with other molecules, which are capable of binding to chloride ions. These cavities have been found to be capable of binding to nucleophilic molecules such as water, ammonia, and hydroxide ions. The binding constants for these reactions are relatively weak, with second-order rate constants on the order of 10 s−1. 4-Nitrothioanisole also reacts with nitric acid to produce nitronium ion and HNO2. This reaction can be used to determine the concentration of chloride ions in solution by measuring the absorbance at 190 nm.