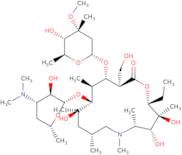
Azithromycin impurity D
CAS: 612069-26-8
Rif. 3D-FA63632
| 1mg | Fuori produzione | ||
| 2mg | Fuori produzione | ||
| 5mg | Fuori produzione | ||
| 10mg | Fuori produzione | ||
| 25mg | Fuori produzione |
Informazioni sul prodotto
- 1-Oxa-6-azacyclopentadecan-15-one, 13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-14-(hydroxymethyl)-3,5,6,8,10,12-hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-, (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-
- (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-14-(hydroxymethyl)-3,5,6,8,10,12-hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one
- Azithromycin F
Azithromycin impurity D is a granulation by-product of azithromycin. It is used as an excipient in the production of intravenous microparticles, which are used to deliver antibiotics to the liver. Research has shown that calcitriol, a form of vitamin D, may lower levels of azithromycin impurity D and therefore reduce its side effects. Azithromycin impurity D is also a crystalline compound with an ester function and glyceride chain. It has a high viscosity and can be used as a substitute for other excipients such as poloxamer in the production of microparticles.