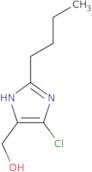
2-Butyl-5-chloroimidazole-4-methanol
CAS: 79047-41-9
Rif. 3D-FB19499
| 1g | Fuori produzione | ||
| 2g | Fuori produzione | ||
| 5g | Fuori produzione | ||
| 250mg | Fuori produzione | ||
| 500mg | Fuori produzione |
Informazioni sul prodotto
- 2-Butyl-4-chloro-5-(hydroxymethyl)imidazole2-n-Butyl-4-chloro-5-(hydroxymethyl)imidazole5-Chloro-2-butyl-imidazole-4-methanol
- (2-Butyl-5-chloro-1H-imidazol-4-yl)methanol
- (2-butyl-4-chloro-1H-imidazol-5-yl)methanol
- 1H-Imidazole-4-methanol, 2-butyl-5-chloro-
- 2-Butyl-4-chloro-5-hydroxymethyl-1H-imidazole
- 2-Butyl-4-chloroimidazole-5-methanol
- 2-Butyl-5-chloro-1H-imidazole-4-methanol
- 2-n-Butyl-4-chloro-5-hydroxymethyl imidazole
- 5-Chloro-2-butylimidazole-4-methanol
- 2-Butyl-4-chloro-5-(hydroxymethyl)imidazole
- Vedi altri sinonimi
2-Butyl-5-chloroimidazole-4-methanol (BICM) is an antagonist of the angiotensin II receptor. It is a prodrug that is metabolized to a reactive intermediate, which binds reversibly to the receptor. The affinity of BICM for the angiotensin II receptor has been shown to be dependent on the regiochemistry of the imidazole ring and on the substituents at positions 2 and 5. This compound was found to have high affinity for receptors with a phenyl substituent at position 5, but low affinity for those with methyl substituents at positions 2 and 5. In vitro activity has been demonstrated in experiments involving binding studies in calf thymus DNA and bacterial DNA gyrase, as well as in dna synthesis and protein synthesis. A conformational study has also been conducted by comparing BICM with biphenyl.