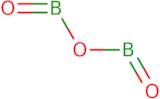
Boron trioxide - min 99.98% trace metals basis
CAS: 1303-86-2
Rif. 3D-FB41577
| 5g | Fuori produzione | ||
| 10g | Fuori produzione | ||
| 25g | Fuori produzione | ||
| 50g | Fuori produzione | ||
| 100g | Fuori produzione |
Informazioni sul prodotto
- B 108406
- Boracic anhydride
- Boria
- Boria (B2O3)
- Boria (B<sub>2</sub>O<sub>3</sub>)
- Boric acid (HBO2), anhydride
- Boric acid (HBO<sub>2</sub>), anhydride
- Boric acid anhydride
- Boric oxide
- Boric oxide (B2O3)
- Vedi altri sinonimi
- Boric oxide (B<sub>2</sub>O<sub>3</sub>)
- Boron oxide
- Boron oxide (B2O3)
- Boron oxide (B<sub>0.67</sub>O)
- Boron oxide (B<sub>2</sub>O<sub>3</sub>)
- Boron oxide (BO<sub>1.5</sub>)
- Boron sesquioxide
- Boron trioxide
- Boron(III) oxide
- Boroxid
- Diboron Trioxide
- Dibortrioxid
- Dioxodiboroxane
- Fused boric acid
- Trioxido De Diboro
- Trioxyde de dibore
Boron trioxide is a white solid that can be produced from the reaction of boric acid and sodium hydroxide. It has the chemical formula B2O3, a basic structure and water vapor as a by-product. Boron trioxide can also be produced by heating boric acid with phosphorus pentoxide or reacting it with hydrogen fluoride. The anhydride form of boron trioxide is used as a model system in electrochemical impedance spectroscopy. Boron trioxide is not toxic to plants and animals, but has been shown to have biological properties such as antifungal properties against Candida albicans and antibacterial properties against Escherichia coli. Boron trioxide has been investigated for its use in zirconium oxide coatings for corrosion protection.