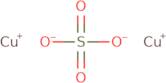
Informazioni sul prodotto
- Copper sulfate (Cu2SO4)
- Copper sulfate (Cu<sub>2</sub>SO<sub>4</sub>)
- Cuprous sulfate
- Dicopper sulfate
- Dicopper(1+) Sulfate
- Sulfuric acid, copper(1+) salt (1:2)
- Sulfuric acid, dicopper(1+) salt
Copper sulfate is a chemical compound that has a redox potential of -0.4 V, which is typical for transition metals. It can be used as a reducing agent and has been shown to react with nitrogen-containing compounds to form copper nitrate. Copper sulfate can be used as an electrochemical catalyst in hydrogenation reactions, such as the reduction of fatty acids, hydroxyl groups, or disulfide bonds. Copper sulfate is soluble in water, but insoluble in most organic solvents. In terms of reactivity, it is acidic and reacts with sodium carbonate to produce carbon dioxide gas. The reaction solution may become cloudy due to the formation of copper(II) carbonate and sulfur dioxide gas.