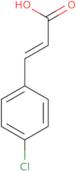
trans-4-Chlorocinnamic acid
CAS: 940-62-5
Rif. 3D-FC20213
| 1g | Fuori produzione | ||
| 2g | Fuori produzione | ||
| 5g | Fuori produzione | ||
| 10g | Fuori produzione | ||
| 25g | Fuori produzione |
Informazioni sul prodotto
- (2E)-3-(4-Chlorophenyl)-2-propenoic acidNSC 52172
- (2E)-3-(4-Chlorophenyl)-2-propenoic acid
- (E)-3-(4-Chlorophenyl)-2-propenoic acid
- (E)-3-(4-Chlorophenyl)acrylic acid
- (E)-4-Chlorocinnamic acid
- 2-Propenoic acid, 3-(4-chlorophenyl)-, (2E)-
- 2-Propenoic acid, 3-(4-chlorophenyl)-, (E)-
- 2-Propenoic acid, 3-(4-chlorophenyl)-, (E)- (9CI)
- Cinnamic acid, p-chloro-, (E)-
- Cinnamic acid, p-chloro-, (E)- (8CI)
- Vedi altri sinonimi
- Cinnamic acid, p-chloro-, trans-
- Nsc 52172
- trans-3-(4-Chlorophenyl)-2-propenoic acid
- trans-p-Chlorocinnamic acid
Trans-4-chlorocinnamic acid is an aromatic hydrocarbon that is a precursor to the important natural compound p-coumaric acid. It is a white crystalline solid with a melting point of 147-149 °C and a boiling point of 338 °C. 4-Chlorocinnamic acid has been shown to inhibit the growth of Candida glabrata, which is an emerging opportunistic fungal pathogen found in immunocompromised patients. Trans-4-chlorocinnamic acid reacts with tyrosinase to form a cinnamate intermediate, which undergoes oxidation reactions that produce reactive oxygen species and quinone radicals. These reactive intermediates cause damage to the cell membrane, leading to cell death. The inhibition of tyrosinase by trans-4-chlorocinnamic acid has been shown using model systems and chemical reactions in vitro.