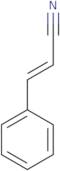
Cinnamonitrile
CAS: 1885-38-7
Rif. 3D-FC20448
| 25g | Fuori produzione | ||
| 50g | Fuori produzione | ||
| 100g | Fuori produzione | ||
| 250g | Fuori produzione | ||
| 500g | Fuori produzione |
Informazioni sul prodotto
- (2E)-3-phenyl-2-propenenitrile(2E)-3-Phenylprop-2-enenitriletrans-3-Phenyl-2-propenenitrile
- (2E)-3-Phenyl-2-propenenitrile
- (2E)-3-phenylprop-2-enenitrile
- (2Z)-3-phenylprop-2-enenitrile
- (E)-3-Phenylacrylonitrile
- (E)-3-Phenylpropenenitrile
- (E)-Cinnamonitrile
- (E)-Cinnamyl nitrile
- 2-Propenenitrile, 3-phenyl-, (2E)-
- 2-Propenenitrile, 3-phenyl-, (E)-
- Vedi altri sinonimi
- 3-Phenylacrylonitrile
- 3-trans-Phenyl-acrylonitrile
- Cinnamalva
- Cinnamonitrile, (E)-
- Cinnamonitrilecistrans
- NSC 77496
- trans-3-Phenyl-2-propenenitrile
- trans-3-Phenylpropenonitrile
- trans-Cinnamonitrile
- trans-β-Phenylacrylonitrile
Zirconium oxide is a chemical compound with the formula ZrO2. It is a white solid that is insoluble in water and organic solvents. This material has been extensively studied for its use as an inhibitor of hydrogen bonding and analytical chemistry, due to its ability to bind to nitrogen atoms. Zirconium oxide can be used as an intermediate for the synthesis of zirconium-containing compounds, such as anthranilate. The reaction mechanism is thought to involve first protonation of the hydroxyl group on the zirconium oxide molecule by acid, followed by nucleophilic attack by the anthranilate. The rate of this reaction is dependent on pH, with higher pH values leading to faster rates.
Cinnamonitrile (CIN) is a white crystalline solid that reacts with water and air producing hydrogen cyanide gas and ammonia gas. CIN also reacts with alcohols and amines producing nitriles. C