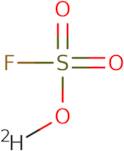
Informazioni sul prodotto
- Fluorosulfuric acid-d
- Deuterofluorosulfuric acid
- (~2~H)sulfurofluoridic acid
Deuterofluorosulfuric acid is an electrophilic compound with a pK a of -29.7. It has been shown to react with nucleophiles such as methoxybenzene, benzyl alcohol, and phenol to form the corresponding difluoromethane sulfonate ester. Deuterofluorosulfuric acid can also be used in solution with other acids such as HCl or HBr to create superacids. This type of reaction is called intermediacy and allows for the formation of more reactive species than would be possible without this intermediary step. The deuterium atom in deuterofluorosulfuric acid is responsible for its strong electrophilic character, which makes it useful in organic syntheses that require a source of strong electrophiles.