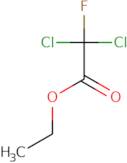
Informazioni sul prodotto
- Acetic acid, 2,2-dichloro-2-fluoro-, ethyl ester
- Acetic acid, dichlorofluoro-, ethyl ester
- Dichlorofluoroacetic acid ethyl ester
- Ethyl 2,2-dichloro-2-fluoroacetate
Ethyl dichlorofluoroacetate is a molecule with a stereochemical configuration that is not found in nature. It has two different configurations, the E and Z, which are mirror images of each other. The two isomers can be distinguished by their spectra, yields, and techniques used to synthesize them. The E configuration of ethyl dichlorofluoroacetate isomerizes to the Z configuration at room temperature. This reaction is reversible and does not have an activation energy barrier. The E configuration of ethyl dichlorofluoroacetate can also be converted to the Z configuration by exposure to light or heat.
Ethanolamine can act as a nucleophile and react with ethyl dichlorofluoroacetate in an S2 reaction to form an intermediate sulfoxide molecule with a pyrrolidino structure. This intermediate reacts with another ethanolamine molecule in an S1 reaction to form the final product, 3-ethyl-2-chlor