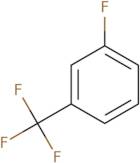
3-Fluorobenzotrifluoride
CAS: 401-80-9
Rif. 3D-FF64594
| 1kg | Fuori produzione | ||
| 2kg | Fuori produzione | ||
| 100g | Fuori produzione | ||
| 250g | Fuori produzione | ||
| 500g | Fuori produzione |
Informazioni sul prodotto
- (2,6-Difluoropyridin-4-Yl)Boronic Acid
- 1-Fluoro-3-(trifluoro-methyl)benzene
- 1-Fluoro-3-(trifluoromethyl)benzene
- 3-Fluoro(trifluoromethyl)benzene
- 3-Fluoro-Α,Α,Α-Trifluoro Toluene
- Benzene, 1-fluoro-3-(trifluoromethyl)-
- Meta Fluoro Benzotrifluoride
- NSC 10315
- Toluene, m,α,α,α-tetrafluoro-
- Trifluoromethyl-3-fluorobenzene
- Vedi altri sinonimi
- alpha,alpha,alpha,3-Tetrafluorotoluene
- m,α,α,α-Tetrafluorotoluene
- m-(Trifluoromethyl)fluorobenzene
- m-Fluoro(trifluoromethyl)benzene
- m-Fluorobenzotrifluoride
- α,α,α,3-Tetrafluorotoluene
3-Fluorobenzotrifluoride is a fluorinating agent that reacts with organic compounds in the liquid phase to produce fluorinated products. The reaction is initiated by adding silver ions, which cause the release of fluorine from the 3-fluorobenzotrifluoride molecule. 3-Fluorobenzotrifluoride is a strong electron acceptor and can react with nitrate ion to form nitrosyl fluoride (NOF). The reaction mechanism involves the initial formation of an oxo radical on the benzene ring. The radical then reacts with NOF to form a hydroperoxyl radical, which abstracts hydrogen from benzene to form a perhydrobenzofuran radical. This intermediate reacts with NOF to produce a fluoroalkane and generates hydrogen peroxide as a byproduct.