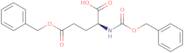
Z-glu(OBzl)-OH NA
CAS: 5680-86-4
Rif. 3D-FG38037
| 5g | Fuori produzione | ||
| 10g | Fuori produzione | ||
| 25g | Fuori produzione | ||
| 50g | Fuori produzione | ||
| 100g | Fuori produzione |
Informazioni sul prodotto
- (2S)-5-(benzyloxy)-2-{[(benzyloxy)carbonyl]amino}-5-oxopentanoic acid (non-preferred name)
- (2S)-5-Oxo-5-phenylmethoxy-2-(phenylmethoxycarbonylamino)pentanoic acid
- (S)-2-Benzyloxycarbonylamino-pentanedioic acid 5-benzyl ester
- 5-(Benzyloxy)-2-{[(Benzyloxy)Carbonyl]Amino}-5-Oxopentanoic Acid (Non-Preferred Name)
- 5-(Phenylmethyl) hydrogen N-[(phenylmethoxy)carbonyl]-<span class="text-smallcaps">L</span>-glutamate
- <span class="text-smallcaps">L</span>-Glutamic acid, N-[(phenylmethoxy)carbonyl]-, 5-(phenylmethyl) ester
- Carbobenzoxy-<span class="text-smallcaps">L</span>-glutamic acid γ-benzyl ester
- Cbz-Glu(OBzl)-OH
- Cbz-L-Glutamic Acid-r-Benyzl Ester
- Cbz-L-glutamic acid 5-benzyl ester
- Vedi altri sinonimi
- Glutamic acid, N-carboxy-, N,5-dibenzyl ester, <span class="text-smallcaps">L</span>-
- N-(Benzyloxycarbonyl)-<span class="text-smallcaps">L</span>-glutamic acid γ-benzyl ester
- N-CBZ-L-Glutamic Acid Gamma-Benzyl Ester
- N-Carbobenzoxy-<span class="text-smallcaps">L</span>-glutamic acid 5-benzyl ester
- N-Carbobenzoxy-<span class="text-smallcaps">L</span>-glutamic acid γ-benzyl ester
- N-Carbobenzyloxy-<span class="text-smallcaps">L</span>-glutamic acid γ-benzyl ester
- N-a-CBZ-(g-OBzl)-D-Glu
- NSC 169178
- Z-Glutamic Acid-O Benzyl Ester
- γ-Benzyl N-(benzyloxycarbonyl)-<span class="text-smallcaps">L</span>-glutamate
- L-Glutamic acid, N-[(phenylmethoxy)carbonyl]-, 5-(phenylmethyl) ester
- Glutamic acid, N-carboxy-, N,5-dibenzyl ester, L-
- γ-Benzyl N-(benzyloxycarbonyl)-L-glutamate
- 5-(Phenylmethyl) hydrogen N-[(phenylmethoxy)carbonyl]-L-glutamate
- N-Carbobenzoxy-L-glutamic acid 5-benzyl ester
NA is a hydrophobic molecule that has been synthesised to be prebiotic. It has been shown to have high specificity for the amination reaction, which is a synthetic reaction. NA can also be synthesised from sequences of pre-existing polypeptides, and homochiral NA can be obtained by using the rotator technique. The reaction time for the synthesis of NA is short, and it can be activated with l-glutamic acid or norvaline. The biological properties of NA are still being studied, but it has been shown to have anti-inflammatory activities in vitro.