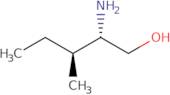
Informazioni sul prodotto
- L-Ile-ol(S)-2-Amino-3-methyl-1-pentanol
- (2S,3S)-1-hydroxy-3-methylpentan-2-aminium
- (2S,3S)-2-Amino-3-methyl-1-pentanol
- (2S,3S)-2-amino-3-methylpentan-1-ol
- (2S,3S)-Isoleucinol
- (S)-Isoleucinol
- (S)-sec-Leucinol
- 1-Pentanol, 2-amino-3-methyl-, (2S,3S)-
- 1-Pentanol, 2-amino-3-methyl-, <span class="text-smallcaps">L</span>-erythro-
- 1-Pentanol, 2-amino-3-methyl-, [S-(R*,R*)]-
- Vedi altri sinonimi
- 2-Amino-3-Methylpentan-1-Ol
- <span class="text-smallcaps">L</span>-Isoleucinol
- [(1S,2S)-1-(Hydroxymethyl)-2-methylbutyl]amine
- [(2S,3S)-1-Hydroxy-3-methylpentan-2-yl]amine
- [(S,S)-1-(Hydroxymethyl)-2-methylbutyl]amine
- 1-Pentanol, 2-amino-3-methyl-, L-erythro-
Isoleucinol is a hydroxylated form of leucine. It is a diagnostic agent that can be used to determine the levels of atp in cells. Isoleucinol binds to pluronic p123 and reacts with trifluoroacetic acid (TFA) to form the corresponding L-isoleucinol-TFA adduct, which is stable at neutral pH. The adduct can be detected by preparative high performance liquid chromatography (HPLC). Isoleucinol can also be synthesized from fatty acids or hydrochloric acid. The synthesis proceeds in two steps: first, the isoleucine side chain is removed by a dehydrating enzyme, and then an alcohol group is added by an oxidoreductase enzyme. This synthetic process produces an enantiomerically pure product, which can be used as a chiral building block for asymmetric synthesis.