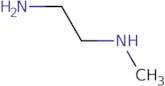
N-Methylethylenediamine
CAS: 109-81-9
Rif. 3D-FM32184
| 5g | Fuori produzione | ||
| 10g | Fuori produzione | ||
| 25g | Fuori produzione | ||
| 50g | Fuori produzione | ||
| 100g | Fuori produzione |
Informazioni sul prodotto
- 2-Methylaminoethylamine
- (2-Aminoethyl)methylamine
- 1,2-Ethanediamine, N-methyl-
- 1,2-Ethanediamine, N<sup>1</sup>-methyl-
- 1-(Methylamino)-2-aminoethane
- 1-Methylethylenediamine
- 2-(Methylamino)ethanamine
- 2-(Methylamino)ethylamine
- Ethylenediamine, N-methyl-
- N-Methyl-1,2-diaminoethane
- Vedi altri sinonimi
- N-Methyl-1,2-ethylenediamine
- N-methyl-1,2-Ethanediamine
- N-methylethane-1,2-diamine
- N-methylethane-1,2-diaminium
- N<sup>1</sup>-Methyl-1,2-ethanediamine
- 1,2-Ethanediamine, N1-methyl-
- N1-Methyl-1,2-ethanediamine
- N1-methylethane-1,2-diamine
N-Methylethylenediamine (NMEA) is a primary amine that can act as a nucleophile in reactions with electron-rich substrates. It is used as a fluorescent probe to detect the presence of nitrogen atoms in proteins and DNA. NMEA can be used to generate fluorescence by reacting with an electron-rich substrate, such as tryptophan, and has been shown to inhibit the proliferation of fibroblast cells. The reaction mechanism of NMEA involves attack by the nitrogen atom on the carbon atom of an electron-rich substrate, forming an intermediate that undergoes hydrolysis to form a carbonyl group or other derivative.
The equilibrium constant for this reaction was found to be 10.2 at 25°C (298 K). In x-ray crystal structures of heterocyclic amines, NMEA was found to have a linear plot when plotted against wavelength. This indicates that there are no changes in electronic transitions