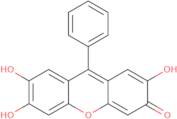
Phenylfluorone
CAS: 975-17-7
Rif. 3D-FP102969
| 1g | Fuori produzione | ||
| 250mg | Fuori produzione | ||
| 500mg | Fuori produzione |
Informazioni sul prodotto
- 2,3,7-Trihydroxy-9-phenyl-6-fluorone
- 2,3,7-Trihydroxy-9-phenylxanthen-6-one
- 2,6,7-Trihydroxy-9-Phenylxanthen-3-One
- 2,6,7-Trihydroxy-9-phenylisoxanthen-3-one
- 2,6,7-trihydroxy-9-phenyl-3H-xanthen-3-one
- 3H-Xanthen-3-one, 2,6,7-trihydroxy-9-phenyl-
- 9-Phenyl-2,3,7-trihydroxy-6-flurone
- 9-Phenyl-3-fluorone
- Fluorone Black
- Fluorone, phenyl-
- Vedi altri sinonimi
- NSC 2608
- NSC 66463
- 9-Phenyl-2,3,7-trihydroxy-6-fluorone
Phenylfluorone is a cetylpyridinium-based anion that has been used as a chemical probe for the study of redox potentials. Phenylfluorone is stable in the solid phase microextraction (SPME) technique, which is based on the extraction of volatile compounds from a surface by adsorption to a sorbent. This compound can be used as an analytical tool for measuring plasma mass spectrometry and fluorescence spectrometry. Phenylfluorone reacts with malonic acid to form phenylmalonic acid, which changes its fluorescence spectrum. The reaction mechanism of phenylfluorone with malonic acid is shown below:
Phenylfluorone + Malonic Acid → Phenylmalonic Acid
Redox Potential = 0.67 V
H2O2 + 2e- → H2O
Redox Potential = -0.