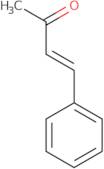
trans-4-Phenyl-3-buten-2-one
CAS: 1896-62-4
Rif. 3D-FP175838
| 1kg | Fuori produzione | ||
| 2kg | Fuori produzione | ||
| 100g | Fuori produzione | ||
| 250g | Fuori produzione | ||
| 500g | Fuori produzione |
Informazioni sul prodotto
- (3E)-4-Phenyl-3-buten-2-one
- (E)-4-Phenyl-3-Buten-2-One
- (E)-Benzalacetone
- (E)-Benzylideneacetone
- (E)-Methyl styryl ketone
- 3-Buten-2-one, 4-phenyl-, (3E)-
- 3-Buten-2-one, 4-phenyl-, (E)-
- BAR
- Methyl (E)-2-phenylethenyl ketone
- Methyl trans-styryl ketone
- Vedi altri sinonimi
- Tc-Bar
- Trans-4-Fenilbut-3-En-2-Ona
- Trans-4-Phenylbut-3-En-2-One
- Trans-4-Phenylbut-3-Ene-2-One
- trans-1-Phenylbut-1-en-3-one
- trans-4-Phenyl-3-butene-2-one
- trans-4-Phenylbut-3-en-2-on
- trans-4-Phenylbuten-2-one
- trans-Benzalacetone
- trans-Benzylideneacetone
- trans-Phenylvinyl methyl ketone
The compound trans-4-Phenyl-3-buten-2-one is a xanthene derivative that belongs to the group of pyridones. It is an inhibitor of cytochrome P450 enzymes, which are responsible for metabolizing foreign substances in the body. Trans-4-Phenyl-3-buten-2-one inhibits the enzyme activity of CYP1A1 and CYP1A2. The mechanism of action has been shown by in vitro assays, electrochemical impedance spectroscopy and transfer reactions to be due to hydroxylation at the 4 position. This hydroxylation leads to an increased concentration of reactive intermediates, which attack nearby electron rich molecules such as DNA or proteins. Transferred electrons lead to signal pathways and structural analysis, which are important for understanding how these compounds interact with their biological targets. The basic structure is a heterocyclic aromatic ring with two double bonds on opposite carbons and one