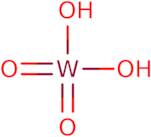
Tungstic acid
CAS: 7783-03-1
Rif. 3D-FT01338
| 25g | Fuori produzione | ||
| 50g | Fuori produzione | ||
| 100g | Fuori produzione | ||
| 250g | Fuori produzione | ||
| 500g | Fuori produzione |
Informazioni sul prodotto
- Dihydrogen Wolframate
- Dihydrogen tetraoxotungstate(2-)
- Dihydrogenwolframat
- Tungstate (WO42-), hydrogen (1:2), (T-4)-
- Tungstate (WO<sub>4</sub><sup>2-</sup>), dihydrogen, (T-4)-
- Tungstate (WO<sub>4</sub><sup>2-</sup>), hydrogen (1:2), (T-4)-
- Tungstate de dihydrogene
- Tungsten acid (H2WO4)
- Tungsten acid (H<sub>2</sub>WO<sub>4</sub>)
- Tungsten hydroxide oxide (W(OH)2O2)
- Vedi altri sinonimi
- Tungsten hydroxide oxide (W(OH)<sub>2</sub>O<sub>2</sub>)
- Tungsten hydroxide oxide (W(OH)<sub>2</sub>O<sub>2</sub>), (T-4)-
- Tungstic acid (H2WO4)
- Tungstic acid (H<sub>2</sub>WO<sub>4</sub>)
- Tungstic(VI) acid
- Volframato De Dihidrogeno
- dihydrogen tungstate (H2WO4)
- dihydrogen tungstate (H<sub>2</sub>WO<sub>4</sub>)
- Tungstate (WO42-), dihydrogen, (T-4)-
Tungstic acid is a solid acidic oxide that is used as an oxidation catalyst for organic reactions. Tungstic acid can also be used in the production of p-hydroxybenzoic acid, which is an intermediate in the synthesis of vitamin C. Tungsten has been shown to catalyze the reaction between hydrogen fluoride and water vapor with a molar ratio of 1:2 to produce hydrogen tungstate. The reaction mechanism is thought to involve intermolecular hydrogen bonding that leads to the formation of particles at high temperatures and a phase transition temperature of about 500°C. Tungsten has good chemical stability in air and does not decompose or react with many substances.