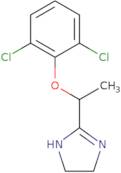
Lofexidine
CAS: 31036-80-3
Rif. 3D-GBA03680
| 1mg | Fuori produzione | ||
| 10mg | Fuori produzione | ||
| 25mg | Fuori produzione | ||
| 50mg | Fuori produzione | ||
| 100mg | Fuori produzione | ||
| 250mg | Fuori produzione |
Informazioni sul prodotto
- 1H-Imidazole, 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-
- (±)-Lofexidine
- 2-[1-(2,6-Dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole
- 2-(1-(2,6-Dichlorophenoxy)Ethyl)-4,5-Dihydro-1H-Imidazole
- 2-Imidazoline, 2-[1-(2,6-dichlorophenoxy)ethyl]-
- 2-[1-(2,6-Dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole
- 1H-imidazole, 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-
- 2-{1-[(2,6-dichlorophenyl)oxy]ethyl}-4,5-dihydro-1H-imidazole
- (±)-2-[1-(2,6-Dichlorophenoxy)ethyl]-2-imidazoline
- 2-(a-(2,6-Dichlorophenoxy)ethyl)2-imidazoline
- Vedi altri sinonimi
Lofexidine is a centrally acting α2 adrenergic agonist that has been used for the treatment of opioid withdrawal. It binds to presynaptic α2-adrenergic receptors and decreases the release of norepinephrine from nerve endings, which helps to reduce withdrawal symptoms. Lofexidine also has an effect on bowel motility, which may be due to its ability to inhibit the release of acetylcholine in the myenteric plexus. Lofexidine is metabolized by cytochrome P450 enzymes in the liver and excreted in urine as metabolites or unchanged drug. Lofexidine should not be taken with clonidine because it may cause excessive hypotension. Lofexidine can also cause a number of adverse effects, including headache, nausea, vomiting, dizziness, dry mouth, constipation and urinary retention.