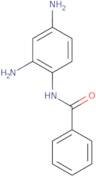
N-(2,4-Diaminophenyl)benzamide
CAS: 56120-01-5
Rif. 3D-GCA12001
| 1g | Fuori produzione | ||
| 5g | Fuori produzione | ||
| 100mg | Fuori produzione | ||
| 250mg | Fuori produzione | ||
| 500mg | Fuori produzione |
Informazioni sul prodotto
The hydrolysis of N-(2,4-diaminophenyl)benzamide (DAB) is a reversible process. In the presence of acid, DAB is protonated and the amide groups are ionized. The rate constant for this reaction is dependent on pH and can be affected by changing chemical conditions such as temperature and solvent. DAB hydrolysis is a kinetic process that has been studied at different concentrations. It has been shown that the hydrolysis rate constants are proportional to the concentration of DAB in solution. The rate constant for DAB hydrolysis follows an Arrhenius equation with an activation energy of about 36 kJ/mol. Hydrolysis reactions are important for DAB because they lead to its degradation and subsequent removal from cells, tissues, or other living organisms.