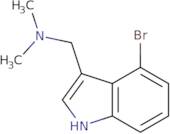
4-Bromogramine
CAS: 64258-88-4
Rif. 3D-PCA25888
| 1g | Fuori produzione | ||
| 5g | Fuori produzione | ||
| 10g | Fuori produzione | ||
| 25g | Fuori produzione | ||
| 100mg | Fuori produzione | ||
| 500mg | Fuori produzione |
Informazioni sul prodotto
Bromination reactions are chemical reactions in which a bromine atom is added to an organic compound in the presence of a catalyst. These reactions are typically carried out with bromine and an organic solvent such as dichloromethane or chloroform, although other reagents can be used. There are two main types of bromination reactions: electrophilic and nucleophilic. Electrophilic brominations occur when the organic substrate is protonated by the base catalyst, while nucleophilic brominations involve the attack of a nucleophile on the electrophile. Bromination reactions can result in regioselectivities, depending on the type of reaction. One example of regioselectivity is that electrophilic brominations will produce one major product when a methyl group is present in the substrate, while nucleophilic brominations will give different products depending on whether there is a hydrogen atom or not. Bromograms are techniques used to study reactivity