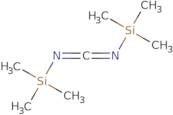
Bis(trimethylsilyl)carbodiimide
CAS: 1000-70-0
Ref. 3D-FB75604
| 2g | Descontinuado | ||
| 5g | Descontinuado | ||
| 10g | Descontinuado | ||
| 25g | Descontinuado | ||
| 50g | Descontinuado |
Informação sobre produto
- 1,3-Bis(trimethylsilyl)carbodiimide
- 2,2,6,6-Tetramethyl-3,5-diaza-2,6-disilahepta-3,4-diene
- Brn 2042082
- Carbodiimide, bis(trimethylsilyl)-
- Carbodiimide, bis(trimethylsilyl)- (7CI,8CI)
- N,N'-Methane tetraylbis(1,1,1-trimethylsilanamine)
- N,N'-Methanetetraylbis(1,1,1-trimethylsilylamine)
- N,N'-bis(trimethylsilyl)carbodiimide
- N,N′-Methanetetraylbis[1,1,1-trimethylsilanamine]
- Silanamine, N,N'-methane tetraylbis(1,1,1-trimethyl-
- Ver mais sinónimos
- Silanamine, N,N'-methanetetraylbis(1,1,1-trimethyl-
Bis(trimethylsilyl)carbodiimide (BTSC) is a carbodiimide that has been used extensively in the chemical industry as a reagent for the synthesis of urea- and thiourea-containing polymers. It is also used to prepare immobilized dyes and fluorescent probes, to coat surfaces, and to catalyze chemical reactions. BTSC reacts with hydrogen fluoride (HF) at room temperature by forming hydrogen gas and bis(trimethylsilyl)urea, which can be quantified using gravimetric analysis. Bis(trimethylsilyl)carbodiimide reacts rapidly with chlorine gas (Cl2), yielding dichlorocarbene, which can be quantified using luminescence properties. The reaction of BTSC with silicon dioxide (SiO2) yields Si-N bonds that are useful in coating applications.
Bis(trimethylsilyl)carbodiim