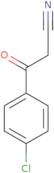
Informação sobre produto
- 4-Chloro-b-oxo-benzenepropanenitrile3-(4-Chlorophenyl)-3-oxopropanenitrile3-Oxo-3-(4-chlorophenyl)propionitrile
- 3-(4-Chlorophenyl)-3-oxopropanenitrile
- 3-(4-Chloro-phenyl)-3-oxo-propionitrile
- 3-(4-Chlorphenyl)-3-oxopropanonitril
- Benzenepropanenitrile, 4-chloro-beta-oxo-
- 4-Chlorophenyl cyanomethyl ketone
- 4-Chlorophenacylcyanide
4-Chlorobenzoylacetonitrile is an efficient method for the synthesis of aldehydes from formyl compounds. It has been used to synthesize a variety of organic compounds, including drugs, pharmaceuticals, and natural products. This reaction takes place in three steps: (1) addition of thiourea to the formyl compound; (2) conversion of the thiourea to a thiol derivative; and (3) treatment with molybdenum trioxide. The second step is stereoselective, so it can be used to produce either R or S-enantiomers. The final product is an aldehyde with a formyl group at the alpha position. In this reaction mechanism, hydrogen bonds are broken by cleaving a C-H bond adjacent to an active methylene group. The reaction is initiated by irradiation or heating due to the release of energy that is stored in these bonds.