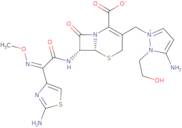
Cefoselis
CAS: 122841-10-5
Ref. 3D-FC44042
| 1mg | A consultar | ||
| 2mg | A consultar | ||
| 5mg | A consultar | ||
| 10mg | A consultar | ||
| 25mg | A consultar |
Informação sobre produto
- (-)-5-Amino-2-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-(2-hydroxyethyl)pyrazolium hydroxide, inner salt, 7<sup>2</sup>-(Z)-(O-methyloxime)
- (6R,7R)-3-{[5-amino-1-(2-hydroxyethyl)-1H-pyrazol-2-ium-2-yl]methyl}-7-{[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
- (6R,7R)-7-[[(2Z)-2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-3-[[2,3-dihydro-2-(2-hydroxyethyl)-3-imino-1H-pyrazol-1-yl]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-[[2,3-dihydro-2-(2-hydroxyethyl)-3-imino-1H-pyrazol-1-yl]methyl]-8-oxo-, [6R-[6α,7β(Z)]]-
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-[[2,3-dihydro-2-(2-hydroxyethyl)-3-imino-1H-pyrazol-1-yl]methyl]-8-oxo-, (6R,7R)-
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-3-[[2,3-dihydro-2-(2-hydroxyethyl)-3-imino-1H-pyrazol-1-yl]methyl]-8-oxo-, (6R,7R)-
- 5-amino-2-{[(6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl}-1-(2-hydroxyethyl)-1H-pyrazol-2-ium
- Cefoselis main-ring
- Wincef
Cefoselis is a cephalosporin antibiotic that has been shown to be effective against bacterial infections. It is the only oral cephalosporin that has been approved for the treatment of community-acquired pneumonia. Cefoselis is administered in low doses in order to minimize the risk of developing bacterial resistance. Cefoselis binds to bacterial 16S ribosomal RNA and inhibits protein synthesis, leading to cell death by inhibiting the production of proteins vital for cell division. The reaction mechanism of cefoselis involves the formation of an acylsulfate intermediate, which reacts with nucleophilic sulfur atoms in cysteine residues on the bacterial cell wall. This leads to lysis of the bacterial cell membrane and cell death.
Cefoselis sulfate is a salt form of cefoselis that is stable at high temperatures and can be dissolved in water or human serum without precipitation. It also has a chemical stability that enables it to
Propriedades químicas
Consulta técnica sobre: 3D-FC44042 Cefoselis
Se desejar solicitar um orçamento ou fazer uma encomenda, por favor, adicione os produtos ao seu carrinho e depois solicite um orçamento ou encomenda a partir do carrinho. É mais rápido, mais barato e poderá beneficiar-se dos descontos e outras vantagens disponíveis.