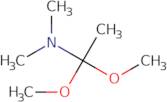
N,N-Dimethylacetamide dimethylacetal
CAS: 18871-66-4
Ref. 3D-FD149151
| 5g | Descontinuado | ||
| 10g | Descontinuado | ||
| 25g | Descontinuado | ||
| 50g | Descontinuado | ||
| 100g | Descontinuado |
Informação sobre produto
- 1,1-Dimethoxyethyl(dimethyl)amine
- (1,1-Dimethoxyethyl)dimethylamine
- 1,1-Dimethoxy-N,N-Dimethylethylamine
- 1,1-Dimethoxy-N,N-dimethylethan-1-amine
- 1,1-Dimethoxyethyl(Dimethyl)Amine
- 1,1-dimethoxy-N,N-dimethylethanamine
- 1,1-dimethoxy-N,N-dimethylethanaminium
- 1-(Dimethylamino)-1,1-dimethoxyethane
- Acetamide, N,N-dimethyl-, dimethyl acetal
- DMF dimethyl acetyl
- Ver mais sinónimos
- Dimethylacetamide dimethyl acetal
- Ethanamine, 1,1-dimethoxy-N,N-dimethyl-
- Ethylamine, 1,1-dimethoxy-N,N-dimethyl-
- N,N' Dimethylacetamide-O, O Dimethyl Acetal
- N,N-Dimethylformamide dimethyl cetal
- N-(1,1-Dimethoxyethyl)-N,N-dimethylamine
- N-(1,1-Dimethoxyethyl)Dimethylamine
- N,N-Dimethylacetamide dimethyl acetal
Dimethylacetamide dimethylacetal is a cationic surfactant that is used as a fluorescent derivative in the study of bacterial morphology. It reacts with hydroxyl groups, thus inhibiting the synthesis of bacteria. This compound also has antimicrobial properties and is effective against methyl ketones. Dimethylacetamide dimethylacetal is synthesized by reacting dodecanol with acetamide, forming an amide bond between the two molecules. The reaction mechanism involves oxygen nucleophiles, such as water or hydroxyl ion, attacking the carbonyl group on the molecule to form a new compound.