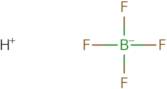
Fluoroboric acid 50% aq
CAS: 16872-11-0
Ref. 3D-FF02671
| 1kg | Descontinuado | ||
| 50g | Descontinuado | ||
| 100g | Descontinuado | ||
| 250g | Descontinuado | ||
| 500g | Descontinuado |
Informação sobre produto
- Acide fluoborique
- Acide tetrafluoroborique
- Acido Tetrafluoroborico
- Acido fluoborico
- Borate(1-), tetrafluoro-, hydrogen (1:1)
- Borate, Tetrafluoro-, Hydrogen
- Borofluoric acid
- Fluoboric acid
- Fluoboric acid (HBF4)
- Fluoboric acid (HBF<sub>4</sub>)
- Ver mais sinónimos
- Fluoborisk syre
- Fluorborsäure
- Fluoroboric acid
- HBF<sub>4</sub>
- Hydrofluoboric acid
- Hydrogen tetrafluoroborate
- Hydrogen tetrafluoroborate(1-)
- Tetrafluorborsäure
- Tetrafluoroborato de hidrógeno
- Tetrafluoroboric Acid
- Tetrafluoroborisk syre
- Tetrafluoroborsaeure
- Tetrafluoroborsaure
- ホウフッ化水素酸
- 四フッ化ホウ酸水素
- Fluoboric acid~Tetrafluoroboric acid
- tetrafluoroborate
- fluoroboronic acid
- trifluoroborane hydrofluoride
- Borate(1-), tetrafluoro-, hydrogen
- fluoroboric acid 50 % solution in water
- Fluoroboric acid 50% solution
Fluoroboric acid is a chemical compound with the molecular formula BF3. It is a strong acid that is soluble in water and other polar solvents. The fluorine atom has three lone pairs of electrons, which makes it a reactive species. Fluoroboric acid can be used to remove metal ions from wastewater, such as magnesium and iron, by forming insoluble salts. Fluoroboric acid also reacts with malonic acid to form tetrafluoroborate, which can be used as a reducing agent in organic synthesis. The crystal structures of fluoroboric acid have been determined using x-ray crystallography. Surface methodology experiments have shown that fluoroboric acid adsorbs well to Langmuir monolayers at low concentrations, but not at high concentrations. Trifluoroacetic acid (TFA) is an organic compound that reacts with hydrogen fluoride to produce trifluoroacetic anhydride (TFAA). TFAA can be used for