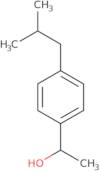
a-(4-Isobutylphenyl)ethanol
CAS: 40150-92-3
Ref. 3D-FI24662
| 25mg | Descontinuado | ||
| 50mg | Descontinuado | ||
| 100mg | Descontinuado | ||
| 250mg | Descontinuado | ||
| 500mg | Descontinuado |
Informação sobre produto
- a-Methyl-4-(2-methylpropyl)benzenemethanol
- (1-Hydroxyethyl)-4-(2-methylpropyl)benzene
- 1-(4-Isobutylphenyl)ethan-1-ol
- 1-(4-Isobutylphenyl)ethanol
- 1-(p-Isobutylphenyl)ethanol
- 1-[4-(2-Methylpropyl)phenyl]ethan-1-ol
- 4-(2-Methylpropyl)-α-methylbenzenemethanol
- Benzene, 1-(1-hydroxyethyl)-4-isobutyl-
- Benzenemethanol, α-methyl-4-(2-methylpropyl)-
- α-(4-Isobutylphenyl)ethanol
- Ver mais sinónimos
- α-(4-Isobutylphenyl)ethyl alcohol
- α-Methyl-4-(2-methylpropyl)benzenemethanol
a-(4-Isobutylphenyl)ethanol is a phenol compound that has been shown to react with hydrochloric acid in a reaction system. The activation energy for the reaction was found to be 40.8 kJ/mol, which is low enough to allow for a surface methodology such as solvation to occur. The solute was found to be anions, which are negatively charged ions that are released by the hydrolysis of organic substances. The chloride ion is one example of an anion and its concentration determines the selectivity of the reaction. The reaction mechanism is a type of functional theory, where two compounds react with each other and form new products. The reaction temperature can range from -80°C to 160°C, depending on what type of organic solvent is used in the process. Photocatalytic activity has been observed when using a-(4-isobutylphenyl)ethanol as the photoactive material.