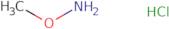o-Methylhydroxylamine HCl
CAS: 593-56-6
Ref. 3D-FM02667
| 5g | Descontinuado | ||
| 10g | Descontinuado | ||
| 25g | Descontinuado | ||
| 50g | Descontinuado | ||
| 100g | Descontinuado | ||
| 250g | Descontinuado |
Informação sobre produto
- (Aminooxy)methane hydrochloride
- Chlorure de methoxyammonium
- Cloruro De Metoxiamonio
- Hydroxylamine methyl ether hydrochloride
- Hydroxylamine, O-methyl-, hydrochloride (1:1)
- Methoxyamine hydrochloride
- Methoxyammonium Chloride
- Methoxyammoniumchlorid
- Methoxylamine Chloride
- Methoxylamine hydrochloride
- Ver mais sinónimos
- N-Methoxylamine hydrochloride
- O-Methoxyamine hydrochloride
- O-Methylhydroxyamine hydrochloride
- O-Methylhydroxylamine hydrochloride
- Mox Reagent
- MHH
- O-Methylhydroxylammonium Chloride
- Hydroxylamine, O-methyl-, hydrochloride
- Mah
- Mox(Tm) Reagent
- methyloxyammonium chloride
- (Aminooxy)Methane Hydrochloride (1:1)
- O-Methylhydroxylamine Hcl
- Methoxylamine HCl
o-Methylhydroxylamine HCl is a compound that has minimal toxicity in animals and humans and is not carcinogenic. It is used to synthesize methoxyamine, which can be used as a control method for the synthesis of pemetrexed. o-Methylhydroxylamine HCl has been shown to induce apoptosis in melanoma cells through the mitochondrial pathway. The compound also inhibits cell growth by inhibiting the enzyme alcohol dehydrogenase, which converts ethanol to acetaldehyde in the presence of NAD+ and molecular oxygen. The structure of o-Methylhydroxylamine HCl was determined using x-ray diffraction data collected at room temperature on a crystal of potassium tert-butoxide with a rate constant of 0.03 s−1. This analytical method was used to determine the purity of o-Methylhydroxylamine HCl as 97%.