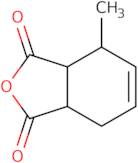
3-Methyltetrahydrophthalic anhydride
CAS: 5333-84-6
Ref. 3D-FM171730
| 1g | Descontinuado | ||
| 2g | Descontinuado | ||
| 100mg | Descontinuado | ||
| 250mg | Descontinuado | ||
| 500mg | Descontinuado |
Informação sobre produto
- 1,2,3,6-Tetrahydro-3-methylphthalic anhydride
- 1,3-Isobenzofurandione, 3a,4,7,7a-tetrahydro-4-methyl-
- 3-Methyl-1,2,3,6-tetrahydrophthalic anhydride
- 3-Methyl-4-cyclohexene-1,2-dicarboxylic acid anhydride
- 3-Methyl-4-cyclohexene-1,2-dicarboxylic anhydride
- 3-Methyl-Δ<sup>4</sup>-tetrahydrophthalic anhydride
- 3-Mthpa
- 3a,4,7,7a-Tetrahydro-4-methyl-1,3-isobenzofurandione
- 4-Cyclohexene-1,2-dicarboxylic anhydride, 3-methyl-
- 4-Methyl-1,2,3,6-tetrahydrophthalic Anhydride
- Ver mais sinónimos
- 4-Methyl-3A,4,5,7A-Tetrahydro-2-Benzofuran-1,3-Dione
- 4-Methyl-3A,4,7,7A-Tetrahydro-2-Benzofuran-1,3-Dione
- 5-Methyl-3A,4,7,7A-Tetrahydro-2-Benzofuran-1,3-Dione
- Ech 202
- I-Mthfa
- NSC 2352
3-Methyltetrahydrophthalic anhydride is a polycarboxylic acid that can be used as a chemical intermediate. It is typically used to synthesize polyvinyl chloride (PVC) by reacting with ethylene. This process requires the use of an organocatalyst, such as titanium tetrachloride, which converts 3-methyltetrahydrophthalic anhydride into vinyl chloride monomer. 3-Methyltetrahydrophthalic anhydride also reacts with other aromatic hydrocarbons in the presence of a catalyst to form alicyclic compounds. The activation energy for this reaction is 31 kJ/mol and the viscosity of 3-methyltetrahydrophthalic anhydride is 1.5 centipoise at 25 degrees Celsius and 1 atmosphere pressure.