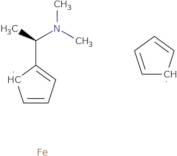
(R)-(+)-N,N-Dimethyl-1-ferrocenylethylamine
CAS: 31886-58-5
Ref. 3D-GBA88658
| 1g | Descontinuado | ||
| 5g | Descontinuado | ||
| 10g | Descontinuado | ||
| 250mg | Descontinuado | ||
| 500mg | Descontinuado |
Informação sobre produto
- (R)-(-)-N,N-Dimethyl-1-ferrocenyl ethylamine
- (R)-(1-N,N-Dimethylaminoethyl)ferrocene
- (R)-1-(Dimethylamino)-1-ferrocenylethane
- (R)-[1-(Dimethylamino)ethyl]ferrocene
- 1-cyclopenta-2,4-dienyl-[2-[(1R)-1-dimethylaminoethyl]-1-cyclopenta-2,4-dienyl]iron
- Ferrocene, [(1R)-1-(dimethylamino)ethyl]-
- Ferrocene, [1-(dimethylamino)ethyl]-, (R)-
- Ferrocenemethylamine, N,N,α-trimethyl-, (R)-(+)-
- Ugi's amine
- [(1R)-1-(Dimethylamino)ethyl]ferrocene
- Ver mais sinónimos
(R)-(+)-N,N-Dimethyl-1-ferrocenylethylamine is a chiral catalyst that is used in the transfer hydrogenation of olefins. It also has asymmetric properties. This agent catalyzes the transfer of one hydrogen atom to an olefinic double bond and can be used for reductive amination reactions. (R)-(+)-N,N-Dimethyl-1-ferrocenylethylamine can be used for the synthesis of esters from acetyl chloride and alcohols, as well as for Friedel-Crafts reactions with aromatic compounds. The mechanism of this process is not fully understood but it is thought that nucleophilic attack by this agent on the carbonyl group leads to formation of an intermediate carbocation that reacts with a second molecule of the alcohol or phenol to form an ester or ketone respectively.