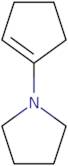
1-Pyrrolidino-1-cyclopentene
CAS: 7148-07-4
Ref. 3D-HAA14807
| 1g | Descontinuado | ||
| 5g | Descontinuado | ||
| 10g | Descontinuado | ||
| 25g | Descontinuado | ||
| 50g | Descontinuado | ||
| 100g | Descontinuado | ||
| 100mg | Descontinuado |
Informação sobre produto
- 1-(1-Cyclopenten-1-yl)pyrrolidine
- 1-(1-Cyclopentenyl)pyrrolidine
- 1-(1-Pyrrolidinyl)-1-cyclopentene
- 1-(1-Pyrrolidinyl)cyclopentene
- 1-(Cyclopent-1-En-1-Yl)Pyrrolidine
- 1-(Cyclopent-1′-enyl)pyrrolidine
- 1-Cyclopent-1-En-1-Ylpyrrolidinium
- 1-Cyclopentenylpyrrolidine
- 1-Pyrrolidinocyclopentene
- 1-Pyrrolidinylcyclopentene
- Ver mais sinónimos
- Cyclopentanone pyrrolidine enamine
- N-(1-Cyclopenten-1-yl)pyrrolidine
- N-(1-Cyclopentenyl)pyrrolidine
- N-(cyclopent-1-ene-1-yl)pyrrolidine
- NSC 29653
- Pyrrolidine, 1-(1-cyclopenten-1-yl)-
The reaction of 1-chloro-1-sulfide with cyclopentene in the presence of pyridine and potassium hydroxide produces 1-pyrrolidino-1-cyclopentene. The reaction is an example of a nucleophilic substitution reaction, which occurs when two molecules interact and a third molecule, which is more reactive than the other two, replaces one of the others. The reaction is thermodynamically favored because it releases heat and does not require a catalyst. This reaction proceeds via resonance stabilization of the intermediate chlorosulfonium ion by conjugation with the pyridine ring. The basic hydrolysis step can be accomplished using sodium hydroxide as a base, which breaks down the salt to generate sulfide gas and potassium chloride. This process is also efficient because it does not require much energy or time to complete.