Informação sobre produto
Volatile Higher Silane
Volatile higher silanes are low temperature, high deposition rate precursors. By appropriate selection of precursor and deposition conditions, silicon deposition can be shifted from amorphous hydrogenated silicon toward microcrystalline silicon structures. As the number of silicon atoms increases beyond two, electrons are capable of sigma-sigma bond conjugation. The dissociative adsorption of two of the three hydrogen atoms on terminal silicon atoms has a lower energy barrier.
ALD Material
Atomic layer deposition (ALD) is a chemically self-limiting deposition technique that is based on the sequential use of a gaseous chemical process. A thin film (as fine as -0.1 Å per cycle) results from repeating the deposition sequence as many times as needed to reach a certain thickness. The major characteristic of the films is the resulting conformality and the controlled deposition manner. Precursor selection is key in ALD processes, namely finding molecules which will have enough reactivity to produce the desired films yet are stable enough to be handled and safely delivered to the reaction chamber.
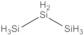
Trisilane; Trisilicane; Silicopropane; Silicon hydride; Trisilicon octahydride
PYROPHORIC?Hform: 121 kJ/mol?Hvap: 27.9 kJ/molBond dissociation energy (Si-Si): 313 kJ/molVapor pressure, 0 °C: 95.5 mmEmployed in low-temperature CVD of silicon and silicon alloysForms silicon nanowires initiated by gold seeds