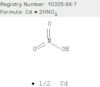
CAS 10325-94-7: Nitric acid, cadmium salt
Description:Nitric acid, cadmium salt, also known as cadmium nitrate, is an inorganic compound with the formula Cd(NO3)2. It typically appears as a white crystalline solid and is highly soluble in water. This compound is characterized by its hygroscopic nature, meaning it can absorb moisture from the air. Cadmium nitrate is primarily used in various applications, including as a precursor in the production of cadmium-based pigments, in electroplating, and in the manufacture of certain types of batteries. It is important to note that cadmium and its compounds are toxic and pose significant health and environmental risks, necessitating careful handling and disposal. The compound can release toxic fumes when heated or in contact with strong acids, and exposure can lead to serious health issues, including respiratory problems and kidney damage. Therefore, appropriate safety measures, including the use of personal protective equipment and proper ventilation, are essential when working with cadmium nitrate.
Formula:Cd.2HNO3
InChI:InChI=1S/Cd.HNO3/c;2-1(3)4/h;(H,2,3,4)
InChI key:InChIKey=AJVCUHHHRPBRHU-UHFFFAOYSA-N
SMILES:[Cd].O=N(=O)O
- Synonyms:
- Cadmium Nitrate
- Cadmium dinitrate
- Cadmium nitrate (Cd(NO3)2)
- Cadmium nitrate (Cd(NO<sub>3</sub>)<sub>2</sub>)
- Cadmium(II) dinitrate
- Cadmium(II) nitrate
- Cadmiumnitrat
- Nitrate de cadmium
- Nitrato De Cadmio
- Nitric acid, cadmium salt (2:1)
- See more synonyms
| Brand | Product data | Purity | Price range | Estimated delivery |
|---|---|---|---|---|
 | Reagecon Cadmium Standard for Atomic Absorption (AAS) 10,000 µg/mL (10,000 ppm) in 1M Nitric Acid (HNO₃) REF: 07-AACDMCAS: 10325-94-7 | - - - | 119.00 € | Mon 31 Mar 25 |

Reagecon Cadmium Standard for Atomic Absorption (AAS) 10,000 µg/mL (10,000 ppm) in 1M Nitric Acid (HNO₃)
Ref: 07-AACDM
| 500ml | 119.00 € |