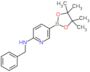
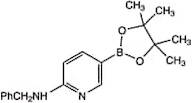
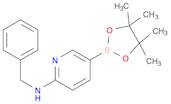
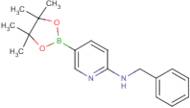
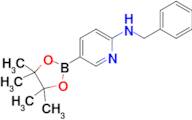
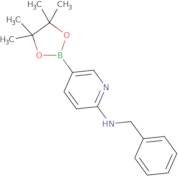
CAS 1073354-27-4: N-benzyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amine
Description:N-benzyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amine is a chemical compound characterized by its unique structure, which includes a pyridine ring substituted with an amine group and a benzyl moiety, as well as a dioxaborolane group. The presence of the dioxaborolane moiety suggests potential applications in organic synthesis, particularly in cross-coupling reactions, due to its ability to participate in boron-mediated transformations. This compound is likely to exhibit moderate to high polarity due to the presence of both polar amine and dioxaborolane functional groups, which may influence its solubility in various solvents. Additionally, the tetramethyl groups contribute to steric hindrance, potentially affecting its reactivity and interaction with other molecules. The compound's molecular structure may also impart specific biological activities, making it of interest in medicinal chemistry. As with many boron-containing compounds, it may exhibit unique properties such as Lewis acidity, which can be exploited in catalysis or material science applications.
Formula:C18H23BN2O2
InChI:InChI=1/C18H23BN2O2/c1-17(2)18(3,4)23-19(22-17)15-10-11-16(21-13-15)20-12-14-8-6-5-7-9-14/h5-11,13H,12H2,1-4H3,(H,20,21)