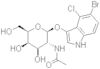
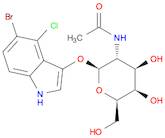
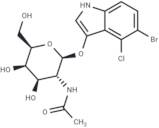
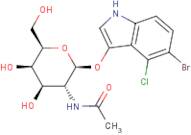
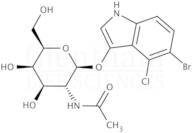
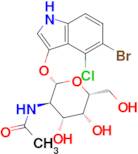
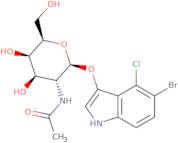
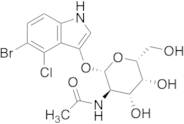
CAS 129572-48-1: 5-bromo-4-chloro-3-indolyl-N-acetyl-B-D-galactosaminide
Description:5-Bromo-4-chloro-3-indolyl-N-acetyl-B-D-galactosaminide, with the CAS number 129572-48-1, is a synthetic compound commonly used as a substrate in biochemical assays, particularly in the study of glycosylation and enzyme activity. This compound features an indole ring, which is known for its aromatic properties and biological significance, particularly in the context of tryptophan derivatives. The presence of bromine and chlorine substituents indicates potential reactivity and specificity in enzymatic reactions. The N-acetyl-B-D-galactosaminide moiety suggests that it can be involved in glycosidic bond formation, making it relevant for studying glycosyltransferases. Its solubility characteristics typically allow for use in aqueous solutions, facilitating its application in various biological assays. Overall, this compound serves as a valuable tool in molecular biology and biochemistry, aiding in the understanding of carbohydrate metabolism and enzyme function.
Formula:C16H18BrClN2O6
InChI:InChI=1/C16H18BrClN2O6/c1-7(22)20-12-14(24)13(23)10(5-21)25-15(12)26-16(17)6-19-9-4-2-3-8(18)11(9)16/h2-4,6,10,12-15,21,23-24H,5H2,1H3,(H,20,22)/t10-,12-,13+,14-,15?,16?/m1/s1
- Synonyms:
- 5-Bromo-4-chloro-3-indolyl N-acetyl-beta-D-galactosaminide
- N-Acetyl-5-bromo-4-chloro-3-indolyl--D-galactosaminide
- 5-bromo-4-chloro-1H-indol-3-yl 2-(acetylamino)-2-deoxy-beta-D-glucopyranoside
- 5-bromo-4-chloro-1H-indol-3-yl 2-(acetylamino)-2-deoxy-beta-D-galactopyranoside