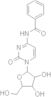
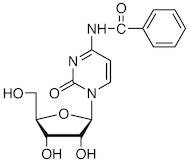
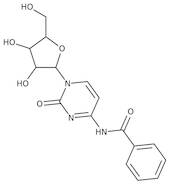
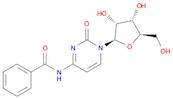
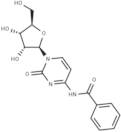
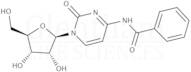
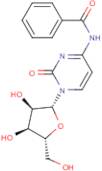
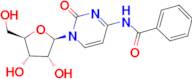
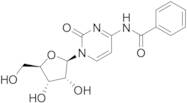
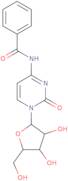
CAS 13089-48-0: N4-benzoylcytidine
Description:N4-benzoylcytidine is a modified nucleoside that features a benzoyl group attached to the nitrogen atom at the 4-position of the cytidine base. This compound is characterized by its structural components, which include a pyrimidine ring and a ribose sugar moiety, typical of nucleosides. The presence of the benzoyl group enhances its lipophilicity and may influence its biological activity, making it of interest in various biochemical and pharmaceutical applications. N4-benzoylcytidine can participate in nucleic acid synthesis and may serve as a substrate or inhibitor in enzymatic reactions involving nucleotides. Its unique structure allows for potential interactions with nucleic acid targets, which can be explored in the context of drug design and development. Additionally, the compound's stability and solubility characteristics are important for its practical applications in research and therapeutic contexts. Overall, N4-benzoylcytidine represents a significant modification of the natural nucleoside cytidine, with implications for molecular biology and medicinal chemistry.
Formula:C16H17N3O6
InChI:InChI=1S/C16H17N3O6/c20-8-10-12(21)13(22)15(25-10)19-7-6-11(18-16(19)24)17-14(23)9-4-2-1-3-5-9/h1-7,10,12-13,15,20-22H,8H2,(H,17,18,23,24)/t10-,12-,13-,15-/m1/s1
InChI key:InChIKey=BNXBRFDWSPXODM-BPGGGUHBSA-N
SMILES:O=C1N=C(C=CN1C2OC(CO)C(O)C2O)NC(=O)C=3C=CC=CC3
- Synonyms:
- 4-(benzoylamino)-1-pentofuranosylpyrimidin-2(1H)-one
- Benzamide, N-(1,2-dihydro-2-oxo-1-β-<span class="text-smallcaps">D</span>-ribofuranosyl-4-pyrimidinyl)-
- Cytidine, N-benzoyl-
- N-benzoyl cytidine
- N-benzoylcytidine
- N6-benzoyl cytosine
- N<sup>4</sup>-Benzoylcytidine
- N<sup>6</sup>-Benzoylcytidine
- NSC 242979