CAS 13933-17-0: Copper, diaquadichloro-
Description:Copper, diaquadichloro- is a coordination compound featuring copper as the central metal ion, coordinated with two water molecules (aqua ligands) and two chloride ions (chloro ligands). This compound typically exhibits a square planar geometry, which is common for copper(II) complexes due to the d9 electron configuration of the copper ion. The presence of both water and chloride ligands contributes to its solubility in water and its potential reactivity in various chemical environments. The compound may display characteristic colors, often associated with copper complexes, which can range from blue to green depending on the specific ligands and their arrangement. Additionally, it may participate in redox reactions, acting as either an oxidizing or reducing agent depending on the conditions. Its applications can span across fields such as catalysis, materials science, and biochemistry, where copper complexes are often utilized for their unique electronic and catalytic properties. Safety and handling precautions should be observed due to the potential toxicity of copper compounds.
Formula:Cl2CuH4O2
InChI:InChI=1S/2ClH.Cu.2H2O/h2*1H;;2*1H2/q;;+2;;/p-2
InChI key:InChIKey=MPTQRFCYZCXJFQ-UHFFFAOYSA-L
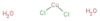
SMILES:[Cl-][Cu+2]([Cl-])([OH2])[OH2]
- Synonyms:
- Copper Chloride Dihydrate
- Copper(2+) Chloride Hydrate (1:2:2)
- Copper(2+) Dichloride
- Copper, diaquadichloro-
- Copper, dichlorodiaquo-
- Cupric Chloride
- Cupric chloride dihydrate
- Cupric(Ⅱ)chloride dihydrate
- Dichlorocopper Dihydrate
- Dichlorocopper Hydrate
- See more synonyms
| Brand | Product data | Purity | Price range | Estimated delivery |
|---|---|---|---|---|
 | Cupric chloride dihydrate REF: 3D-FC165332CAS: 13933-17-0 | Min. 95% | - - - | Discontinued product |

Cupric chloride dihydrate
Ref: 3D-FC165332
| Undefined size | Discontinued | Request information |