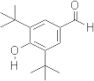
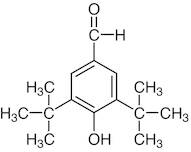
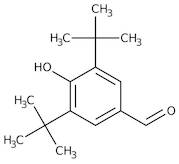
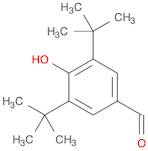
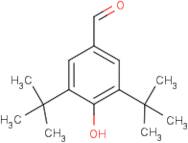
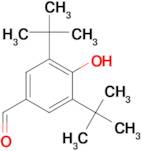
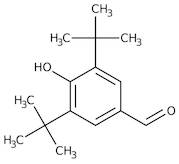
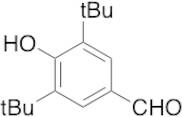
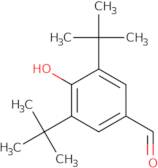
CAS 1620-98-0: 3,5-Di-tert-butyl-4-hydroxybenzaldehyde
Description:3,5-Di-tert-butyl-4-hydroxybenzaldehyde, with the CAS number 1620-98-0, is an organic compound characterized by its aromatic structure featuring a hydroxyl group and an aldehyde functional group. It is a derivative of hydroxybenzaldehyde, specifically substituted with two tert-butyl groups at the 3 and 5 positions of the benzene ring, which significantly enhances its lipophilicity and steric hindrance. This compound is typically a white to pale yellow solid at room temperature and is known for its antioxidant properties, making it useful in various applications, including as a stabilizer in plastics and other materials. The presence of the hydroxyl group contributes to its reactivity, allowing it to participate in various chemical reactions, such as oxidation and esterification. Additionally, its bulky tert-butyl groups can influence its solubility and interaction with other molecules, making it a subject of interest in both synthetic and industrial chemistry. Safety data should be consulted for handling and storage, as with any chemical substance.
Formula:C15H22O2
InChI:InChI=1S/C15H22O2/c1-14(2,3)11-7-10(9-16)8-12(13(11)17)15(4,5)6/h7-9,17H,1-6H3
InChI key:InChIKey=DOZRDZLFLOODMB-UHFFFAOYSA-N
SMILES:O=CC=1C=C(C(O)=C(C1)C(C)(C)C)C(C)(C)C
- Synonyms:
- 2,6-Di-tert-Butyl-4-formylphenol
- 3,5-Bis(1,1-dimethylethyl)-4-hydroxybenzaldehyde
- 3,5-Ditert-butyl-4-hydroxybenzaldehyde
- 3,5-di0T0butyl-4-hydroxybenzaldehyde*hemihydrate
- 4-Formyl-2,6-di-tert-butylphenol
- 4-Hydroxy-3,5-di-tert-butylbenzaldehyde
- Benzaldehyde, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-
- Benzaldehyde, 3,5-di-tert-butyl-4-hydroxy-
- NSC 14450