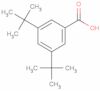
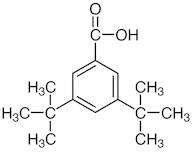
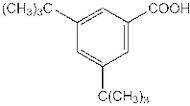
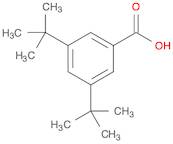
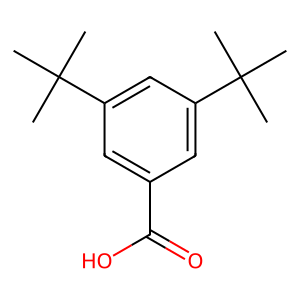
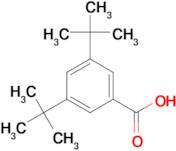
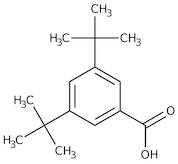
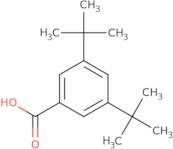
CAS 16225-26-6: 3,5-Bis(1,1-dimethylethyl)benzoic acid
Description:3,5-Bis(1,1-dimethylethyl)benzoic acid, also known by its CAS number 16225-26-6, is an aromatic carboxylic acid characterized by the presence of two tert-butyl groups attached to the benzene ring at the 3 and 5 positions. This compound exhibits typical properties of benzoic acids, including the ability to form hydrogen bonds due to its carboxylic acid functional group, which contributes to its solubility in polar solvents. The bulky tert-butyl groups enhance its hydrophobic character, potentially affecting its solubility in non-polar solvents. The presence of these substituents can also influence the compound's melting and boiling points, as well as its reactivity, making it less prone to oxidation compared to simpler benzoic acids. Additionally, 3,5-Bis(1,1-dimethylethyl)benzoic acid may exhibit applications in various fields, including as an intermediate in organic synthesis or as an additive in polymer formulations, due to its thermal stability and steric hindrance properties.
Formula:C15H22O2
InChI:InChI=1S/C15H22O2/c1-14(2,3)11-7-10(13(16)17)8-12(9-11)15(4,5)6/h7-9H,1-6H3,(H,16,17)
InChI key:InChIKey=NCTSLPBQVXUAHR-UHFFFAOYSA-N
SMILES:O=C(O)C=1C=C(C=C(C1)C(C)(C)C)C(C)(C)C
- Synonyms:
- 3,5-Bis(1,1-dimethylethyl)benzoic acid
- 3,5-Di-T-Butylbenzoic Acid
- 3,5-Ditert-butylbenzoic acid
- Benzoic acid, 3,5-bis(1,1-dimethylethyl)-
- Benzoic acid, 3,5-di-tert-butyl-
- Dibutylbenzoicacid
- 3,5-Di-tert-butylbenzoic acid