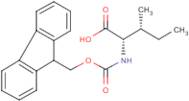
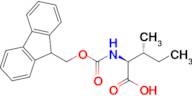
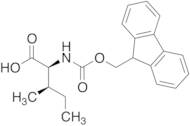
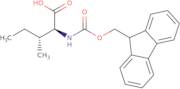
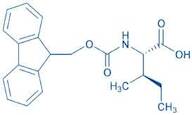
CAS 251316-98-0: N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-alloisoleucine
Description:N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-alloisoleucine, commonly referred to as Fmoc-L-alloisoleucine, is a derivative of the amino acid alloisoleucine, modified with a fluorenylmethoxycarbonyl (Fmoc) protecting group. This compound is primarily utilized in peptide synthesis as a protective group for the amino functionality, allowing for selective reactions without interference from the amine. The Fmoc group is known for its stability under basic conditions and can be removed under mild acidic conditions, making it advantageous for sequential peptide synthesis. The structure of Fmoc-L-alloisoleucine includes a bulky fluorenyl group, which contributes to its steric properties and can influence the conformation of peptides during synthesis. Additionally, the presence of the alloisoleucine side chain, which is an isomer of isoleucine, imparts unique characteristics to the resulting peptides, potentially affecting their biological activity and interactions. Overall, this compound is significant in the field of organic chemistry and biochemistry, particularly in the development of peptide-based therapeutics.
Formula:C21H23NO4
InChI:InChI=1S/C21H23NO4/c1-3-13(2)19(20(23)24)22-21(25)26-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,13,18-19H,3,12H2,1-2H3,(H,22,25)(H,23,24)/t13-,19+/m1/s1
InChI key:InChIKey=QXVFEIPAZSXRGM-YJYMSZOUSA-N
SMILES:O=C(OCC1C=2C=CC=CC2C=3C=CC=CC31)NC(C(=O)O)C(C)CC
- Synonyms:
- (2S,3R)-2-(9H-Fluoren-9-ylmethoxycarboylamino)-3-methylpentanoic acid
- <span class="text-smallcaps">L</span>-Alloisoleucine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-
- Fmoc-<span class="text-smallcaps">L</span>-allo-Ile-OH
- Fmoc-L-alloisoleucine
- N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-<span class="text-smallcaps">L</span>-alloisoleucine
- N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-alloisoleucine
- L-Alloisoleucine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-
- Fmoc-L-allo-Ile-OH








![N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-alloisoleucine](https://static.cymitquimica.com/cas-image/thumb-xs/15407-n-9h-fluoren-9-ylmethoxy-carbonyl-l-alloisoleucine.jpg)






![L-Alloisoleucine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-](https://static.cymitquimica.com/products/IN/thumb/DA002QL2.jpg)