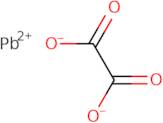
Product Information
- Ethanedioic acid, lead(2+) salt (1:1)
- Lead oxalate (PbC<sub>2</sub>O<sub>4</sub>)
- Lead(2+) Ethanedioate
- Lead(2+) oxalate
- Oxalic acid, lead(2+) salt (1:1)
Lead(II) oxalate is a compound that has the chemical formula PbCO3. It is a low-energy, low-reactive metal hydroxide that reacts with hydrochloric acid to form lead(II) chloride and water. Reaction of lead(II) oxalate with an oxalate salt produces lead(II) carbonate, which can be precipitated by adding a sodium hydroxide solution. Lead oxalate can be obtained from reaction of lead with an acidic environment such as sulphuric acid or phosphoric acid. Lead oxalate is soluble in water and insoluble in organic solvents such as benzene or ethanol. The reaction between lead oxide and hydrochloric acid is the most common method for obtaining this compound. Lead oxalate decomposes at high temperatures and in the presence of light, releasing toxic fumes of lead chloride and carbon dioxide.
Chemical properties
Technical inquiry about: 3D-AAA81493 Lead(II) oxalate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.