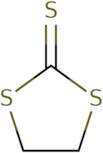
Product Information
- 4,5-Dihydro-1,3-Dithiole-2-thione
- Brn 0106330
- Carbonic acid, trithio-, cyclic ethylene ester
- Cyclic ethylene trithiocarbonate
- Ethylene glycol, cyclic trithiocarbonate
- Nsc 6746
- Trithiocarbonic acid, cyclic ethylene ester
Ethylene trithiocarbonate (ETC) is an organic compound that has the chemical formula CH2=C(S)CS. It is a colorless liquid with a boiling point of 155 ° C. The formation rate of ETC can be increased by adding carbon disulphide to the reaction as a solvent. Methyl anthranilate, amines, and thione compounds are all known to react with ethylene in order to form ETC.
The synthesis of ETC begins with the reaction between ethylene and carbon disulfide. This reaction produces a molecule of CS2 and two molecules of hydrogen sulfide gas. These gases are then allowed to react with methyl anthranilate, which produces ETACS2 and methyl sulfide gas. The ETACS2 is then oxidized using oxygen to form ETACOS2, which is finally reacted with ammonia or methylamine to produce ETACONH or ETAMINE
Chemical properties
Technical inquiry about: 3D-AAA82238 Ethylene trithiocarbonate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.