Product Information
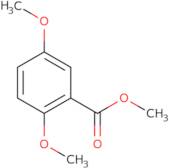
- Benzoic acid, 2,5-dimethoxy-, methyl ester
Methyl 2,5-dimethoxybenzoate is a molecule with a molecular weight of 180.2 g/mol. It can be synthesized by reacting methyl acetate with dimethoxybenzene in the presence of hydrochloric acid and warming the reaction mixture to 80°C. This compound has been shown to have an interaction with diphenyl ethers, proton magnetic resonance, and fluorescence spectroscopy. Methyl 2,5-dimethoxybenzoate has also been found to bind metal ions such as copper(II) and zinc(II). The carboxyl group on this molecule is responsible for coordination of these metals. Mass spectrometric analysis has revealed that Methyl 2,5-dimethoxybenzoate has a mass of 180.2 g/mol.
Chemical properties
Technical inquiry about: 3D-CAA15040 Methyl 2,5-dimethoxybenzoate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.