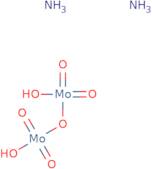
Ammonium dimolybdate
CAS: 27546-07-2
Ref. 3D-CBA54607
| 1kg | To inquire | ||
| 5kg | To inquire | ||
| 100g | To inquire | ||
| 250g | To inquire | ||
| 500g | To inquire |
Product Information
- Ammonium di-molybdate
- Ammonium molybdate ((NH<sub>4</sub>)<sub>2</sub>Mo<sub>2</sub>O<sub>7</sub>)
- Ammonium molybdate (di), 85% (Assay), Mo 56.5%
- Ammonium molybdate(VI) ((NH<sub>4</sub>)<sub>2</sub>Mo<sub>2</sub>O<sub>7</sub>)
- Ammonium molybdenum oxide ((NH<sub>4</sub>)<sub>2</sub>Mo<sub>2</sub>O<sub>7</sub>)
- Ammoniumdimolybdatewhitepowder
- Diammonium dimolybdate
- Diammonium heptaoxodimolybdate
- Diammonium heptaoxodimolybdate(2-)
- Molybdate (Mo<sub>2</sub>O<sub>7</sub><sup>2-</sup>), diammonium
- See more synonyms
- Molybdic acid (H<sub>2</sub>Mo<sub>2</sub>O<sub>7</sub>), diammonium salt
- Molybdic acid (H<sub>4</sub>Mo<sub>4</sub>O<sub>14</sub>), tetraammonium salt
- Ammonium molybdate(VI) ((NH4)2Mo2O7)
- Molybdic acid (H2Mo2O7), diammonium salt
- Ammonium molybdenum oxide ((NH4)2Mo2O7)
- Molybdate (Mo2O72-), diammonium
Ammonium dimolybdate is a molybdenum compound that acts as an oxidation catalyst. It is used in the production of anhydrous sodium, which is used to manufacture aluminum. Ammonium dimolybdate can be synthesized by reacting anhydrous sodium with ammonium and particle molybdenum. This reaction produces ammonium chloride and benzyl groups, which are removed by washing the catalyst with water. Ammonium dimolybdate has a low energy requirement and exothermic reaction, making it useful for a variety of chemical reactions including inorganic acid production.
Chemical properties
Technical inquiry about: 3D-CBA54607 Ammonium dimolybdate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.