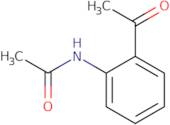
N-(2-Acetyl-phenyl)-acetamide
CAS: 5234-26-4
Ref. 3D-FA150554
| 1g | Discontinued | ||
| 2g | Discontinued | ||
| 100mg | Discontinued | ||
| 250mg | Discontinued | ||
| 500mg | Discontinued |
Product Information
- 2′-(Acetylamino)acetophenone
- 2′-(N-Acetylamino)acetophenone
- 2′-Acetamidoacetophenone
- 2′-Acetylacetanilide
- Acetanilide, 2-acetyl-
- N-(o-Acetylphenyl)acetamide
- NSC 12469
- acetamide, N-(2-acetylphenyl)-
- o-Acetamidoacetophenone
N-(2-Acetylphenyl)acetamide is a substrate for the one-electron reduction of β-unsaturated aldehydes with sodium periodate. This reaction produces the corresponding 2-acetylphenol (2AP). This compound is also a good substrate for the reduction of α,β-unsaturated ketones, including acetophenone and benzophenone. The 2AP can be converted to its corresponding alcohol by using potassium t-butoxide in methanol. These reactions are catalyzed by hydrogen or lithium metal and require an oxidizing agent such as oxygen or dioxygenated water. The electron donor must be capable of donating a lone pair of electrons without undergoing oxidation. These reactions are exothermic and produce a Grignard reagent, which can react with nitrous acid to form an N-nitroso compound.
The functional theory explains these reactions as involving nucleophilic attack at the carbonyl carbon atom and elect