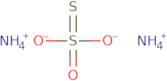
Ammonium thiosulfate
CAS: 7783-18-8
Ref. 3D-FA34437
| 1kg | Discontinued | ||
| 2kg | Discontinued | ||
| 5kg | Discontinued | ||
| 250g | Discontinued | ||
| 500g | Discontinued |
Product Information
- Aminonium Thiosulfate
- Ammonium hyposulfite
- Ammonium thiosulfate ((NH4)2S2O3)
- Ammonium thiosulfate ((NH<sub>4</sub>)<sub>2</sub>S<sub>2</sub>O<sub>3</sub>)
- Ammonium thiosulphate
- Ammoniumthiosulfat
- Ammoniumthiosulfatewhitextl
- Diammonium thiosulfate
- Thio-Sul
- Thiosulfate d'ammonium
- See more synonyms
- Thiosulfuric acid (H2S2O3), ammonium salt (1:2)
- Thiosulfuric acid (H2S2O3), diammonium salt
- Thiosulfuric acid (H<sub>2</sub>S<sub>2</sub>O<sub>3</sub>), ammonium salt (1:2)
- Thiosulfuric acid (H<sub>2</sub>S<sub>2</sub>O<sub>3</sub>), diammonium salt
- Thiosulfuric acid, diammonium salt
- Tiosulfato De Amonio
Ammonium thiosulfate is a water-soluble chemical that is used in wastewater treatment to remove nitrogen from the water. It converts ammonium ions into ammonium sulfate and thiosulfate ions, which are then converted to sulfuric acid and nitrate ions. Ammonium thiosulfate is also used as a fumigant for grain storage. It is activated by mixing with metal hydroxides such as sodium carbonate or calcium hydroxide and reacts with water vapor to form ammonia gas, hydrogen fluoride, and sulfur dioxide. Ammonium thiosulfate can be oxidized by hydrogen peroxide or other oxidizing agents to form sulfuric acid. This chemical has been shown to be an effective protease inhibitor due to its ability to stabilize the protective protein shell of proteases.