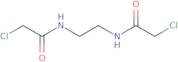
Bischloroacetylethylinediamine
CAS: 2620-09-9
Ref. 3D-FB18806
| 1g | Discontinued | ||
| 2g | Discontinued | ||
| 5g | Discontinued | ||
| 10g | Discontinued | ||
| 500mg | Discontinued |
Product Information
- N,N'-1,2-Ethanediylbis[2-chloro-acetamide]1,2-Bis(chloroacetamido)ethaneN,N'-Bis(chloroacetyl)-1,2-diaminoethane
- 1,2-Bis(chloroacetamido)ethane
- 2-Chloro-N-(2-((2-chloroacetyl)amino)ethyl)acetamide
- Acetamide, N,N'-1,2-ethanediylbis(2-chloro- (9CI)
- Acetamide, N,N'-ethylenebis(2-chloro- (8CI)
- Acetamide, N,N'-ethylenebis(chloro-
- Acetamide, N,N′-1,2-ethanediylbis[2-chloro-
- Acetamide, N,N′-ethylenebis[2-chloro-
- Brn 1781986
- N,N'-Bis(chloroacetyl)ethylenediamine
- See more synonyms
- N,N'-Ethylene-bis(chloroacetamide)
- N,N'-ethane-1,2-diylbis(2-chloroacetamide)
- N,N′-1,2-Ethanediylbis[2-chloroacetamide]
- N,N′-Bis(chloroacetyl)-1,2-diaminoethane
- N,N′-Bis(chloroacetyl)-1,2-ethylenediamine
- N-[2-[(2-Chloroacetyl)amino]ethyl]-2-chloroacetamide
- Nsc 49395
- S 106
Bischloroacetylethylinediamine is a surfactant that is used as a byproduct in the synthesis of thiourea. It has a conformation that varies depending on the presence of transition metal ions, and forms an amide with ethyl bromoacetate. The cationic surfactant lithium cation can form an ion pair with phenyl isothiocyanate and bind to the cavity in bischloroacetylethylenediamine. This cavity can be filled with gemini, which are two amide groups, or with a thioether group. Bischloroacetylethylinediamine can also react stepwise to form another amide group.