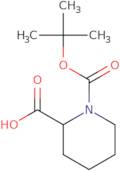
Boc-DL-homoproline
CAS: 98303-20-9
Ref. 3D-FB49704
| 25g | Discontinued | ||
| 50g | Discontinued | ||
| 100g | Discontinued | ||
| 250g | Discontinued | ||
| 500g | Discontinued |
Product Information
- Boc-DL-HomoPro-OHBoc-DL-pipecolic acid
- (2R)-1-(tert-butoxycarbonyl)piperidine-2-carboxylate
- (2S)-1-(tert-butoxycarbonyl)piperidine-2-carboxylate
- N-BOC-Piperidine-2-carboxylic acid
- N-Boc-2-piperidinecarboxylic acid
- 1-N-Boc-2-Piperidinecarboxylic Acid
- 1-(Tert-Butoxycarbonyl)Piperidine-2-Carboxylic Acid
- (+/-)-1-Boc-Piperidine-2-Carboxylic Acid
- Boc-Dl-Pipecolic Acid
- Boc-Dl-Pip-Oh
- See more synonyms
- Boc-Dl-Pic(2)-Oh
- Boc-Dl-Homoproline
- Boc-Dl-Homopro-Oh
- (2R)-1-(tert-butoxycarbonyl)piperidine-2-carboxylic acid
- (R)-1-Boc-piperidine-2-carboxylic acid
- (R)-1-N-Boc-Piperidine-2-Carboxylic Acid
Allylation is a chemical reaction in which an allyl group is added to a molecule, typically with an organometallic reagent such as propylphosphonic acid. In this reaction, the allyl group replaces a hydrogen atom on the substrate molecule. This type of substitution is known as electrophilic addition. The allylamine functional group is one type of carbamate and has three resonance structures. These are shown below:
The first structure shows that the amine nitrogen has two bonds to carbon and one bond to hydrogen. The second structure shows that the amine nitrogen has one bond to carbon and two bonds to hydrogen. The third structure shows that the amine nitrogen has two bonds to carbon and three bonds to hydrogen. This means that there are three possible stereoisomers for allylamines.
Boc-DL-homoproline is a potent agonist for ring-opening reactions, which can be used in organic synthesis for various purposes, including hydro