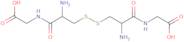
(H-Cys-Gly-OH)2 (Disulfide bond)
CAS: 7729-20-6
Ref. 3D-FC108017
| 25mg | 338.00 € | ||
| 50mg | 365.00 € | ||
| 100mg | 569.00 € |
Product Information
- H-Cys-Gly-OH)2
- (H-Cys-Gly-Oh)2
- 1: PN: IE20060154 PAGE: 25 claimed protein
- 2,2'-{Disulfanediylbis[(2-Amino-1-Oxopropane-3,1-Diyl)Imino]}Diacetic Acid (Non-Preferred Name)
- Bnp 7776S
- Cystinyl-bis-glycine
- Glycine, <span class="text-smallcaps">L</span>-cysteinyl-, bimol. (1→1′)-disulfide
- Glycine, N,N′-<span class="text-smallcaps">L</span>-cystyldi-
- Glycine, N,N′-cystyldi-
- N,N′-<span class="text-smallcaps">L</span>-Cystinyldiglycine
- See more synonyms
- N,N′-<span class="text-smallcaps">L</span>-Cystyldiglycine
- NSC 333711
- Oxidized cysteinylglycine
- Glycine, L-cysteinyl-, bimol. (1→1′)-disulfide
- N,N′-L-Cystinyldiglycine
- N,N′-L-Cystyldiglycine
- Glycine, N,N′-L-cystyldi-
Disulfide bond is a covalent chemical bond that links two sulfur atoms to each other. Disulfides are the major form of sulfur-containing proteins, and are important in many biological processes, such as postnatal development, biliary function, hepatitis diagnosis and cancer. Disulfide bonds can be detected using chromatographic methods such as high performance liquid chromatography (HPLC) or gas chromatography (GC). The biosynthesis of disulfides is an essential process in the metabolism of glutathione. Disulfide bonds are formed by the oxidation of two sulfhydryl groups (-SH) in a protein by reactive oxygen species (ROS), which results in the formation of a single disulfide bond between two cysteine residues. This reaction requires glutathione as a cofactor, which catalyzes the formation of -S-S- bridges between two cysteine residues by reducing them to their corresponding thiols (-SH). Glutathione also
Chemical properties
Technical inquiry about: 3D-FC108017 (H-Cys-Gly-OH)2 (Disulfide bond)
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.