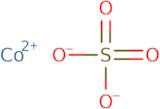
Product Information
- Cobalt Sulphate
- Cobalt monosulfate
- Cobalt sulfate
- Cobalt sulfate (1:1)
- Cobalt sulfate (CoSO4)
- Cobalt sulfate (CoSO<sub>4</sub>)
- Cobalt(2+) sulfate
- Cobalt(II) sulfate
- Cobaltous Sulfate
- Cobaltsulfat
- See more synonyms
- Sulfate de cobalt
- Sulfato De Cobalto
- Sulfuric acid, cobalt (2+) salt
- Sulfuric acid, cobalt(2+) salt (1:1)
Cobalt sulfate is a metal hydroxide that has been used in toxicity studies. It has been shown to have antimicrobial properties and can be used in water treatment to remove sulfate from wastewater. Cobalt sulphate is most effective when the concentration of the compound is between 0.2 and 0.4 M, with optimum concentrations found at about 0.3 M. The solubility of cobalt sulphate increases when it is mixed with sodium citrate, which provides an inexpensive way for the compound to be dissolved in water. Cobalt sulphate can also be removed from water by laser ablation or by heating it to remove water vapor under vacuum conditions. Cobalt sulphate may cause liver lesions in pregnant women and also causes damage to zirconium oxide when exposed to high temperatures.