Product Information
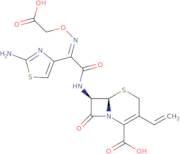
Cefixime impurity D is a contaminant of cefixime that has been found in some batches. It is a trihydrate form of cefixime and has the same antibacterial efficacy as the drug. Cefixime impurity D's presence in pharmaceutical preparations should be detected by control analysis and chromatographic analysis, which can be used to ensure that it does not exceed the prescribed limit. The analytical method for this compound is based on the rate constant and penicillin-binding protein (PBP) binding assay. In order to study its pharmacokinetics, a two-way crossover study was conducted on human volunteers who had streptococcal pharyngitis. The results showed that there were no significant differences between the pharmacokinetic parameters of the trihydrate form and those of cefixime hydrochloride. The wild-type strain of gonorrhea was susceptible to both forms of cefixime, while strains with reduced susceptibility
Chemical properties
Technical inquiry about: 3D-FC63678 Cefixime impurity D
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.